Volume: 13 Issue: 3
Role of Cellular Dissociation Scoring as a Prognostic Factor in Squamous Cell Carcinoma.
Year: 2025, Page: 178-182, Doi: https://doi.org/10.47799/pimr.1303.25.45
Received: Sept. 3, 2025 Accepted: Sept. 9, 2025 Published: Dec. 30, 2025
Abstract
Squamous cell carcinoma is the most common malignancy encountered in the head and neck region. Traditionally it has been graded into well, moderate and poorly differentiated based on the morphological features. Staging plays an important role in the prognosis and thereby survival. The lymph node status is the most important predictor. In recent years, extensive research is going on cellular dissociation grading (CDG) which is based on cell nest size and tumor budding. We have undertaken this study to evaluate cellular dissociation grading and correlate it with lymph node status so as to consider this CDG as an independent prognostic factor in Squamous cell carcinoma.
Keywords: Cellular Dissociation Grade, Score, Squamous cell carcinoma
INTRODUCTION
The cellular dissociation grade is a novel histopathological grading system based on- (a) tumor size budding (tumor budding) and (b) cell nest size. This system has baffled the traditional grading systems which have been used for grading in organs such as lung, head & neck, cervix & esophagus. [1-5] The presence of lymph node metastasis is one of the most important predictors for survival. In the recent years, there is a need to identify additional criteria to avoid unnecessary lymphadenectomies especially in patients who do not benefit from lymph node excision as a part of the treatment. Several studies were done to evaluate tumor budding, a part of cellular dissociation grading as a prognostic factor in colorectal adenocarcinoma. [6-11] The aim of our present study was to evaluate the Cellular Dissociation Grading (CDG) in Squamous cell carcinoma in head & neck region.
Aim
To evaluate role of tumor budding as an individual prognostic factor in Squamous cell carcinoma.
Objectives
-
Calculate the Cellular Dissociation Grading (CDG) scoring based on tumor budding and cell nest size of the tumor.
-
Co relate Cellular Dissociation Grading (CDG) & grading of Squamous cell carcinoma.
-
Compare Cellular Dissociation Grading (CDG) scoring with Lymph node involvement.
MATERIALS & METHODS
Inclusion criteria
-
All the cases diagnosed as Squamous cell carcinoma during the study period.
-
Excision specimens are only included.
-
Cases having Lymph node status with follow up details.
Exclusion criteria
-
Cases diagnosed outside the limit of the study period.
-
Cases without relevant details and follow up.
-
Necrotised and hemorrhagic samples.
-
Small biopsies were not included.
Method
This retrospective study has been undertaken for a period of 3 years, from January 2021 to January 2024. All the cases as per the inclusion criteria were analysed with respect to clinical details including lymph node status. Consent was taken from the patients which was in the form of written consent. Ethical clearance was taken from the ethical committee of the institute after presenting the research study. The H&E slides were prepared. Tumor budding [Fig. 1] & Cell nest size was identified. Tumor bud is defined as single tumor cells or cluster of typically fewer than five cells that have detached from the main tumor mass; found at the invasive front of tumor. Cell nest is a collection of interconnected cells which can be tumor cells or other types of cells; it is rather a general term for any cluster of cells. Prognostic significance: Tumor bud represents an aggressive form of cancer and is associated with higher risk of nodal involvement, metastasis and poor survival rate, whereas the cell nest only when they are small or single cell clusters of pleomorphic cells; it is a prognostic marker of cancer. Based on these 2 parameters, Cellular Dissociation Grading (CDG) scoring was done in all the cases.
| Histological Feature | Classification | Score & Grade |
|---|---|---|
| Score & Grade | No tumor budding | 1 |
| 1–5 tumor buds/HPF | 2 | |
| >5 tumor buds/HPE | 3 | |
| Cell Nest Size (CNS) | >15 cells per nest | Score 1 |
| 5–15 cells per nest | Score 2 | |
| 2–4 cells per nest | Score 3 | |
| single cell invasion | Score 4 | |
| Cellular Dissociation Grade (CDG) | SumTB+CNS=2–3 | Score 1 |
| SumTB+CNS=4–5 | Score 2 | |
| SumTB+CNS=6-7 | Score 3 |
Table 1: Cellular dissociation grading [4-10]
Cellular Dissociation Grading (CDG) scoring: The high-power field with the highest budding activity was chosen for the evaluation.
Well-differentiated tumors had a sum of 2-3 [Fig. 2], moderately differentiated tumors had a sum of 4-5 [Fig. 3] and poorly differentiated tumors had a sum of 6-7 [Fig. 4].
Lymph nodes were resected as a part of the treatment modality in only 11 cases.
All the results were noted in Excel sheets for evaluation of p-value.
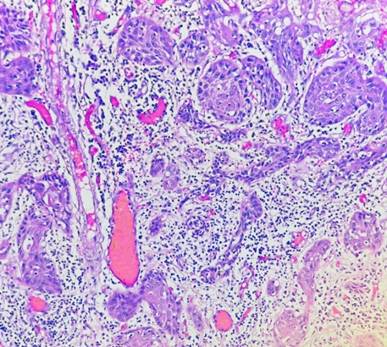
Fig. 1: Tumor buds (Hematoxylin & Eosin: 10X)
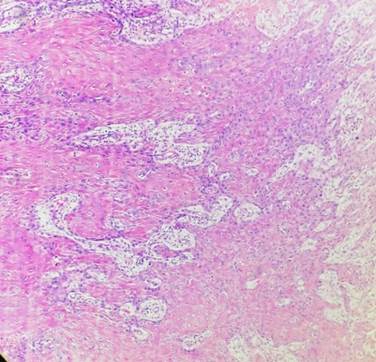
Fig. 2: Cellular Dissociation Grade: Score 1 (Hematoxylin & Eosin 40X)
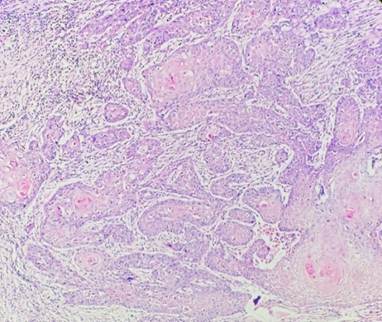
Fig. 3: Cellular Dissociation Grade: Score 2 (Hematoxylin & Eosin 40X)
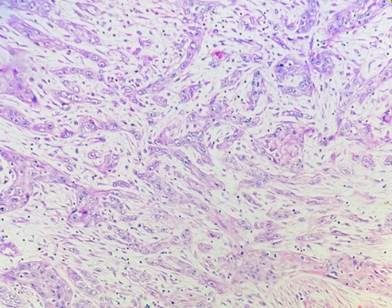
Fig. 4: Cellular Dissociation Grade: Score 3 (Hematoxylin & Eosin 40X)
RESULTS
A total of 32 cases were included in our study.
Age & Sex distribution: Out of 32 cases, 18 were males and remaining were females [Table. 2].
Most of the cases were in the elderly age group, above 60 years with the mean age group of 69.8years [Table. 3].
| Males | Females | |
|---|---|---|
| No. of cases | 18 | 14 |
| % of cases | 56.25% | 43.75% |
Table 2: Sex distribution
| Age distribution | No. of cases | % of cases |
|---|---|---|
| < 40 | 01 | 3.125% |
| 40–50 | 01 | 3.125% |
| 50–60 | 13 | 40.625% |
| 60–70 | 16 | 50% |
| 70–80 | 02 | 6.25% |
Table 3: Age distribution
Majority of the tumors were well differentiated ([Table. 4]).
| Grading of SCC | No. of cases | % of cases |
|---|---|---|
| Well | 18 | 56.25% |
| Moderate | 08 | 25% |
| Poorly | 06 | 18.75% |
Table 4: Grading of tumors
According to grading of the tumor, the cases are divided into well differentiated, moderately differentiated and poorly differentiated. The Cellular dissociation grading is co related with the grading of the tumor; the well differentiated tumors have a less cellular dissociation score when compared to the poorly differentiated tumors [Table. 5].
|
S. No |
Differentiation |
CDG score 1 |
CDG score 2 |
CDG score 3 |
|---|---|---|---|---|
|
1 |
Well |
18(56.25%) |
|
|
|
2 |
Moderate |
04(12.5%) |
04(12.5%) |
|
|
3 |
Poor |
|
02(6.25%) |
04(12.5%) |
Table 5. Differentiation of tumor v/s Cellular Dissociation Grade score
The p value was significant as shown in [Table. 6].
|
|
Differentiation |
Cellular dissociation Grade score |
|---|---|---|
|
Chi square test |
<0.001-0.0021 |
0.001-0.0041 |
|
Univariate analysis |
<0.001-0.006 |
0.001-0.0009 |
Table 6: p value comparing differentiation with cellular dissociation Grade score
During the Staging of the tumors, we have compared the cellular dissociation score with staging of the tumor [Table. 7]. Well differentiated tumors are usually Stage I, most of the moderately differentiated tumors were Stage II and almost all the poorly differentiated tumors were Stage III. The p value was 0.08; the p value was not significant.
|
S. No |
Stage |
Stage I |
Stage II |
Stage III |
|---|---|---|---|---|
|
1 |
Well |
16 |
|
|
|
2 |
Moderate |
|
2 |
6 |
|
3 |
Poor |
|
1 |
5 |
Table 7: Staging of the tumor v/s Cellular Dissociation Grade score
All the cases were compared in respect to the Age, Grade and Cellular dissociation grading [Table. 8].
As seen in the table, well differentiated cases had a lesser cellular dissociation grade and belonged to age group less than 50-60 years whereas poorly differentiated tumors were of age group more than 60 with higher cellular dissociation score. The p value was 0.009 and was insignificant.
| S. No | Age | No. of Cases | Grade | CDG Score Details |
|---|---|---|---|---|
| 1 | <40 | 1 | Well | Score 1 |
| 2 | 40–50 | 1 | Well | Score 1 |
|
3
|
50–60
|
12
|
Well – 8 | Score 1 |
| Moderate – 4 |
2 cases – Score 1 2 cases – Score 2 |
|||
|
4
|
60–70
|
16
|
Well – 7 | Score 1 |
| Moderate – 5 |
3 cases – Score 1 2 cases – Score 2 |
|||
| Poor – 4 |
2 cases – Score 2 2 cases – Score 3 |
|||
| 5 | 70–80 | 2 | Poor | Score 3 |
Table 8: Age versus Grade of the tumor and Cellular Dissociation Grade score
Only 11 cases had undergone lymph node resection. These cases were scored according to the CDG as follows [Table. 9].
| CDG | Nx | N1 |
|---|---|---|
| Score 1 | 1(9%) | |
| Score 2 | 2(18%) | 3(27.5%) |
| Score 3 | 5(45.5%) |
Table 9: CDG scoring versus Lymph node status
The lymph node status were compared with the CDG scoring, Tumor budding & cell nest size. Statistical analysis was done and tabulated as follows [Table. 10].
| Analysis Method | CDG | TB | CNS |
|---|---|---|---|
| Chi square test | <0.001-0.023 | 0.001-0.0047 | 0.004-0.019 |
| Univariate analysis | <0.001-0.009 | 0.001-0.0011 | 0.006-0.022 |
Table 10: Comparison with lymph node status
P value was significant
DISCUSSION
The cellular dissociation grading which takes into consideration the tumor budding and cell nest size has shown to surpass the conventional grading system. [12] In our study we have taken these Two entities i.e. tumor budding and cell nest size were evaluated for the cellular dissociation score was compared with the differentiation or grading of the tumor.. The score is more in poorly differentiated squamous cell carcinoma, and it is less in well differentiated squamous cell carcinoma. These findings corelated with studies done by Derani et al. [12]. All the cases were then compared all the cases with the Staging of the tumor, though most of the well differentiated tumors were of Stage I and the poorly differentiated tumors were Stage III, the p value was not significant. The age of the patients, cellular dissociation score and finally the Staging were all compared. The poorly differentiated tumors which had a greater score belonged to age group above 68 years, but the p value was not significant. This p value was not significant probably due to smaller size of the study. Lymph nodes which were resected in the 11 cases were evaluated and compared to the CDG score. The chi square analysis was done, and the p value was found to be significant. Similar findings were also seen in the study done by Boxberg et al. [1], Jesinghaus et al. [4] and Derani et al. [12]. Staging strategies which may be noninvasive (clinical examination, imaging) or surgical (sentinel lymph node biopsy, staging lymphadenectomy), have a number of limitations like more number of false-negative findings and/or high morbidity rates [13]. In cases of tumor tissue after neoadjuvant chemotherapy, there is a prognostic role for cellular dissociation score as observed in various studies [4, 5]. In our study cellular dissociation score correlates with lymph node status as the p value was significant. Thus, lymph node status also can be taken as an independent prognostic factor apart from cellular dissociation grading.
CONCLUSION
In conclusion cellular dissociation grading had proved to be superior to the traditional grading system in predicting high risk features of the lesion. Cellular dissociation grading has been calculated by taking the cell nest size and tumor budding into consideration. All the well differentiated tumors have less score whereas poorly differentiated tumors have high score, thus this new scoring system can be taken as an independent prognostic factor in Squamous cell carcinoma.
References
1. Boxberg M, Bollwein C, Jöhrens K, Kuhn PH, Haller B, Steiger K, et al. Novel prognostic histopathological grading system in oral squamous cell carcinoma based on tumour budding and cell nest size shows high interobserver and intraobserver concordance. Journal of Clinical Pathology. 2019; 72 (4). Available from: https://doi.org/10.1136/jclinpath-2018-205454
2. Boxberg M, Jesinghaus M, Dorfner C, Mogler C, Drecoll E, Warth A, et al. Tumour budding activity and cell nest size determine patient outcome in oral squamous cell carcinoma: proposal for an adjusted grading system. Histopathology. 2017; 70 (7). Available from: https://doi.org/10.1111/his.13173
3. Boxberg M, Kuhn PH, Reiser M, Erb A, Steiger K, Pickhard A, et al. Tumor Budding and Cell Nest Size Are Highly Prognostic in Laryngeal and Hypopharyngeal Squamous Cell Carcinoma. American Journal of Surgical Pathology. 2019; 43 (3). Available from: https://doi.org/10.1097/pas.0000000000001178
4. Jesinghaus M, Boxberg M, Konukiewitz B, Slotta-Huspenina J, Schlitter AM, Steiger K, et al. A Novel Grading System Based on Tumor Budding and Cell Nest Size Is a Strong Predictor of Patient Outcome in Esophageal Squamous Cell Carcinoma. American Journal of Surgical Pathology. 2017; 41 (8). Available from: https://doi.org/10.1097/pas.0000000000000865
5. Jesinghaus M, Strehl J, Boxberg M, Bruehl F, Wenzel A, Konukiewitz B, et al. Introducing a novel highly prognostic grading scheme based on tumour budding and cell nest size for squamous cell carcinoma of the uterine cervix. The Journal of Pathology: Clinical Research. 2018; 4 (2). Available from: https://doi.org/10.1002/cjp2.95
6. Dawson H, Galuppini F, Träger P, Berger MD, Studer P, Brügger L, et al. Validation of the International Tumor Budding Consensus Conference 2016 recommendations on tumor budding in stage I-IV colorectal cancer. Human Pathology. 2019; 85 Available from: https://doi.org/10.1016/j.humpath.2018.10.023
7. Lugli A, Cathomas G. Pathologie: Tumor Budding beim kolorektalen Karzinom: ein Spitzenspieler auf der Ersatzbank?. Swiss Medical Forum ‒ Schweizerisches Medizin-Forum. 2012; 12 (03). Available from: https://doi.org/10.4414/smf.2012.07746
8. Lugli A, Kirsch R, Ajioka Y, Bosman F, Cathomas G, Dawson H, et al. Recommendations for reporting tumour budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Modern Pathology. 2017; 30 (9). Available from: https://doi.org/10.1038/modpathol.2017.46
9. Müller F, Lugli A, Dawson H. Tumor Budding beim kolorektalen Karzinom – Informationen zur klinischen Anwendung und Anleitung zur praktischen Bestimmung. Der Pathologe. 2022; 43 (1). Available from: https://doi.org/10.1007/s00292-021-01016-6
10. Prall, F. Tumour budding in colorectal carcinoma. Histopathology. 2007; 50 (1). Available from: https://doi.org/10.1111/j.1365-2559.2006.02551.x
11. Prall F, Nizze H, Barten M. Tumour budding as prognostic factor in stage I/II colorectal carcinoma. Histopathology. 2005; 47 (1). Available from: https://doi.org/10.1111/j.1365-2559.2005.02161.x
12. Derani H, Becker AS, Hakenberg O, Erbersdobler A. Evaluation of the Cellular Dissociation Grading, Based on Tumor Budding and Cell Nest Size, in Squamous Cell Carcinoma of the Penis. Cancers. 2022; 14 (19). Available from: https://doi.org/10.3390/cancers14194949
13. Alkatout I, Naumann CM, Hedderich J, Hegele A, Bolenz C, Jünemann KP, et al. Squamous cell carcinoma of the penis: Predicting nodal metastases by histologic grade, pattern of invasion and clinical examination. Urologic Oncology: Seminars and Original Investigations. 2011; 29 (6). Available from: https://doi.org/10.1016/j.urolonc.2009.10.014
Copyright
©2025 (Grandhi) et al. This is an open-access journal, and articles are distributed under the terms of the Creative Commons Attribution License CC-BY 4.0. (https://creativecommons.org/licenses/by/4.0/) which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited.
Cite this article
Grandhi B, Kharidehal D, Nanditha K. Role of Cellular Dissociation Scoring as a Prognostic Factor in Squamous Cell Carcinoma. Perspectives in Medical Research 2025; 13(3):178-182 DOI: 10.47799/pimr.1303.25.45